To stay ahead of the competition, a thorough idea about the competitive landscape, their product range, their strategies, and future prospects are very valuable. Europe Electronic Clinical Outcome Assessment (eCOA) market research report involves a key data and information about the market, emerging trends, product usage, motivating factors for customers and competitors, restraints, brand positioning, and customer behaviour, which is of utmost importance when it comes to achieving a success in the competitive marketplace. This market research report is all-inclusive and encompasses various parameters of the market. Europe Electronic Clinical Outcome Assessment (eCOA) marketing report comprises of the major market insights that takes business to the next level of success and growth.
The significant Europe Electronic Clinical Outcome Assessment (eCOA) business report studies consumption of market, top players involved, sales, price, revenue and market share with volume and value for each region. An analytical assessment of the competitors gives clear idea of the most important challenges faced by them in the current market and in the coming years. Market definition mentioned in this industry report covers the market drivers which indicate the factors causing rise in the market and market restraints which indicates the factors causing fall in the market growth. While preparing Europe Electronic Clinical Outcome Assessment (eCOA) market research report, markets on the local, regional, and global level are explored.
An increasing number of clinical trials, the need to improve compliance, effectively capture and manage clinical information, reduce costs, and increase R&D activities are expected to drive market growth. Kayentis, a French company that specializes in electronic clinical outcome assessment (eCOA) solutions, will receive approximately USD 8.3 million in funding in December 2020. This aided its goals of regional expansion and product development.
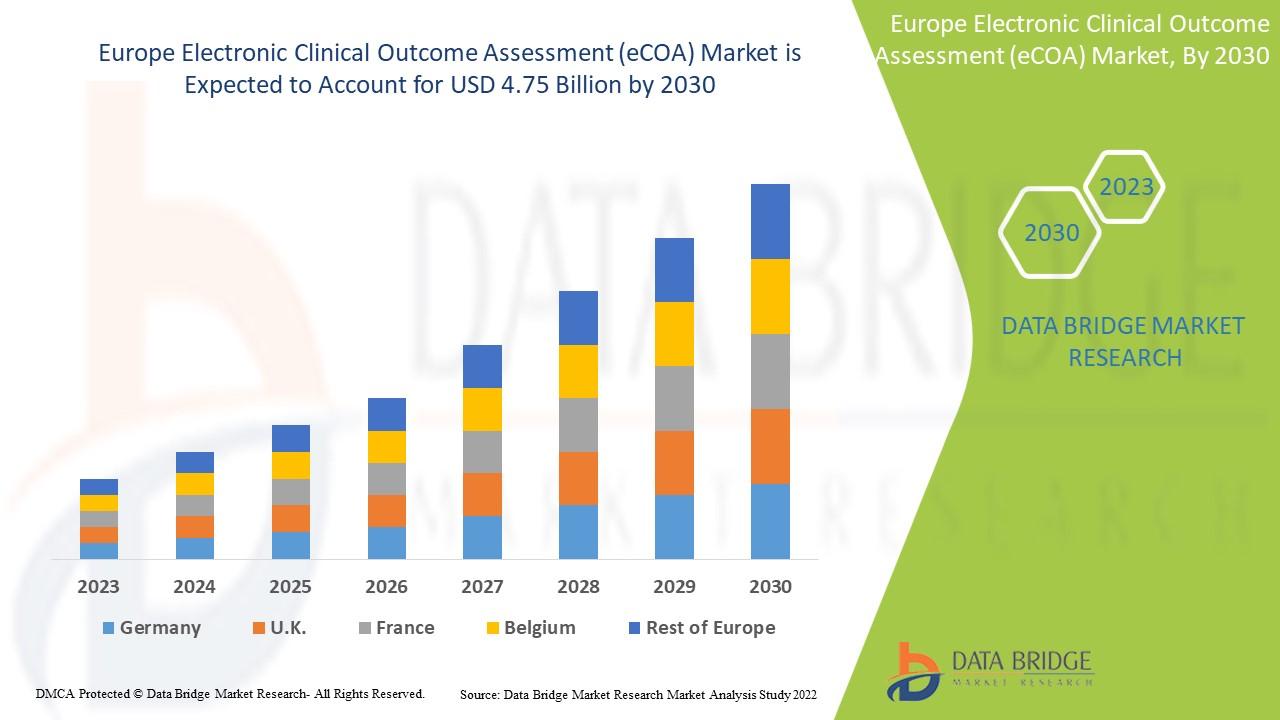
Data Bridge Market Research analyses that the electronic clinical outcome assessment (eCOA) market, which was USD 1.42 billion in 2022, is expected to reach USD 4.75 billion by 2030, at a CAGR of 16.30% during the forecast period 2022 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
To Get a Sample Report, Visit @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=europe-electronic-clinical-outcome-assessment-ecoa-market
Driver:
- Rising burden on pharmaceutical manufacturers
New medication development techniques are increasingly relying on electronic data capture rather than paper-based procedures to reduce total costs. Data collection using electronic clinical outcome assessment platforms improves data quality, harmonises data collection practises, and provides significant value to its users, such as data analysis. Electronic data collection and analysis services eliminate paper-based records' drawbacks while increasing patient compliance. They also reduce the cost of site monitoring and eliminate the risk of data fluctuation. These solutions provide streamlined information, which helps to improve data quality by gathering data in a structured manner. The above-mentioned benefits of using electronic clinical outcome assessments are expected to boost product demand.
- Rise in clinical outcome assessments
Clinical outcome assessments (COAs) are thus being used more frequently to assess the efficacy of various chronic illness therapies. These have frequently been crucial in the regulatory approval of medicines. The addition of numerous services will also help it grow. For instance, in June 2021, Signant Health launched a novel acceleration programme that reduces study setup timelines by 50% or more without compromising clinical data quality. Thus, the increasing launch of novel programmes that benefit companies in assessing clinical trial studies is likely to increase demand for electronic clinical outcome assessment solutions, thereby boosting market growth.
Some key players mentioned in the report are:
IBM Corporation (U.S.), IQVIA (U.S), Medidata Solutions, Inc. (U.S.), Clario (U.S), ArisGlobal (U.S.), Signant Health (U.S.), TransPerfect (U.S.), Cloudbyz (U.S.), Climedo Health GmbH (Germany), ClinCapture (U.S.), Oracle Corporation (U.S.), Paraxel International Corporation (U.S.), eClinical Solutions LLC (U.S.), OmniComm Systems, Inc. (U.S.), CRF Health (U.S.), European Round Table (U.S.)
Key Insights that Study is going to provide:
· The 360-degree Europe Electronic Clinical Outcome Assessment (eCOA) overview based on a global and regional level
· Market Share & Sales Revenue by Key Players & Emerging Regional Players
· A separate chapter on Market Entropy to gain insights on Leaders aggressiveness towards market [Merger & Acquisition / Recent Investment and Key Developments]
· May vary depending upon availability and feasibility of data with respect to Industry targeted
· Patent Analysis** No of patents / Trademark filed in recent years.
· A complete and useful guide for new market aspirants
· Forecast information will drive strategic, innovative and profitable business plans and SWOT analysis of players will pave the way for growth opportunities, risk analysis, investment feasibility and recommendations
· Various Europe Electronic Clinical Outcome Assessment (eCOA) industry leading players are studied with respect to their company profile, product portfolio, capacity, price, cost, and revenue.
Key Market Segmentation:
By Product (On-Premise Solutions, Cloud Based Solutions and Web Based Solutions), Approach (Clinician Reported Outcome Assessment (CLINRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (OBSRO) and Performance Outcome Assessment (PERFO)), End User (Commercial Service Providers, Hospitals and Transplant Centers, Research Laboratories and Academic Institutions)
The countries covered in the Europe Electronic Clinical Outcome Assessment (eCOA) Market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Table of Contents:
Executive Summary
Market Landscape
Market Sizing
Five Forces Analysis
Market Segmentation by End-user
Customer Landscape
Geographic Landscape
Key leading countries
Vendor Landscape
Vendor Analysis
Appendix
To Know More About This Premium Research Report, Visit @ https://www.databridgemarketresearch.com/reports/europe-electronic-clinical-outcome-assessment-ecoa-market
Browse More Trending Reports
https://www.databridgemarketresearch.com/reports/global-prescription-drugs-market
https://www.databridgemarketresearch.com/reports/global-multiple-myeloma-market
https://www.databridgemarketresearch.com/reports/global-cleanroom-technology-market
https://www.databridgemarketresearch.com/reports/asia-pacific-cleanroom-technology-market
https://www.databridgemarketresearch.com/reports/middle-east-and-africa-cleanroom-technology-market
About Data Bridge Market Research:
An absolute way to forecast what future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavours to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:-
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475