The reliable Electronic Trial Master File (eTMF) Systems market report brings into focus public demands, competencies and the constant growth of the working industry, vibrant reporting, or high data protection services while analysing market information. This document highlights across-the-board evaluation of the market’s growth prospects and restrictions. Furthermore, drivers and restraints of the market assessed in this report makes aware about how the product is getting utilized in the recent market environment and also provides estimations about the future practice. Electronic Trial Master File (eTMF) Systems research report is of huge importance in many aspects for better understanding of the market which lead to sky-scraping business growth.
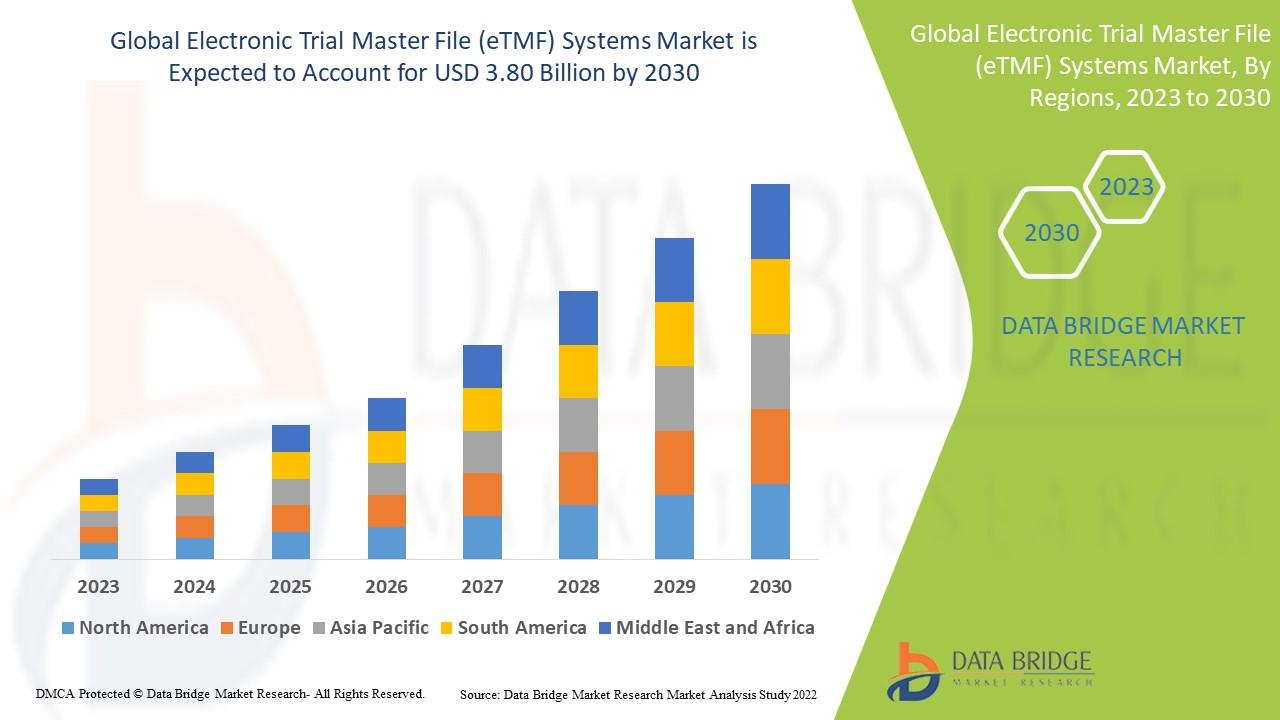
Data Bridge Market Research analyses that the electronic trial master file (eTMF) systems market which was USD 1.44 billion in 2022, is expected to reach USD 3.80 billion by 2030, at a CAGR of 12.9% during the forecast period 2023 to 2030
Download Exclusive Sample Report @
Market Overview
A trial master file in electronic (digital content) format is referred to as an electronic trial master file (eTMF). It is a type of pharmaceutical content management system that provides a formalised method of organising and storing documents, images, and other digital content for pharmaceutical clinical trials that may be required for compliance with government regulatory agencies.
Some of the major players operating in the Electronic Trial Master File (eTMF) Systems are:
IQVIA (U.S.)
· Labcorp Drug Development (U.S.)
· TransPerfect (U.S.)
· Oracle (U.S.)
· Phlexglobal (U.S.)
· SureClinical Inc. (U.S.)
· Aurea, Inc. (U.S.)
· Veeva Systems (U.S.)
· MasterControl, Inc. (U.S.)
· Clinevo Technologies (India)
· Mayo Foundation for Medical Education and Research (MFMER) (U.S.)
· Montrium Inc. (U.S.)
Electronic Trial Master File (eTMF) Systems Scope and Market Size
The electronic trial master file (eTMF) systems market is segmented on the basis of component, delivery mode, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
· IQVIA (U.S.)
· Labcorp Drug Development (U.S.)
· TransPerfect (U.S.)
· Oracle (U.S.)
· Phlexglobal (U.S.)
· SureClinical Inc. (U.S.)
· Aurea, Inc. (U.S.)
· Veeva Systems (U.S.)
· MasterControl, Inc. (U.S.)
· Clinevo Technologies (India)
Browse Full Report Along With Facts and Figures @
Highlights of TOC:
Chapter 1: Market overview
Chapter 2: Global Electronic Trial Master File (eTMF) Systems market
Chapter 3: Regional analysis of the Electronic Trial Master File (eTMF) Systems
Chapter 4: Electronic Trial Master File (eTMF) Systems based on types and applications
Chapter 5: Revenue analysis based on types and applications
Chapter 6: Market share
Chapter 7: Competitive Landscape
Chapter 8: Drivers, Restraints, Challenges, and Opportunities
Chapter 9: Gross Margin and Price Analysis
Browse Trending Reports:
Targeted Protein Degradation Market
About Data Bridge Market Research:
An absolute way to predict what the future holds is to understand the current trend! Data Bridge Market Research presented itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are committed to uncovering the best market opportunities and nurturing effective information for your business to thrive in the marketplace. Data Bridge strives to provide appropriate solutions to complex business challenges and initiates an effortless decision-making process. Data Bridge is a set of pure wisdom and experience that was formulated and framed in 2015 in Pune.
Data Bridge Market Research has more than 500 analysts working in different industries. We have served more than 40% of the Fortune 500 companies Europely and have a network of more than 5,000 clients worldwide. Data Bridge is an expert in creating satisfied customers who trust our services and trust our hard work with certainty. We are pleased with our glorious 99.9% customer satisfaction rating.
Contact Us: -
Data Bridge Market Research
US: +1 888 387 2818
United Kingdom: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: – sopan.gedam@databridgemarketresearch.com