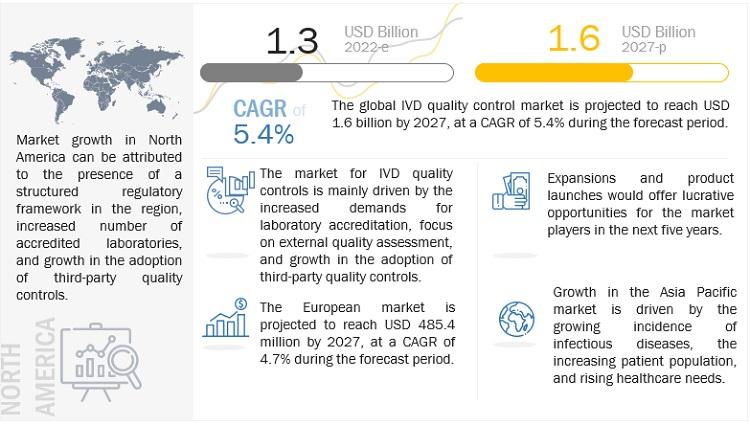
The report "IVD Quality Control Market by Source (Plasma, Whole Blood, Urine), Technology (Immunoassay, Hematology, Microbiology, Coagulation), Manufacturer (Third party, OEM), End Users (Hospitals, Lab, Research) - Global Forecast to 2027", is projected to reach USD 1.6 billion by 2027 from USD 1.3 billion in 2022, at a CAGR of 5.4% during the forecast period.
Browse 254 market data Tables and 54 Figures spread through 331 Pages and in-depth TOC on "IVD Quality Control Market by Source (Plasma, Whole Blood, Urine), Technology (Immunoassay, Hematology, Microbiology, Coagulation), Manufacturer (Third party, OEM), End Users (Hospitals, Lab, Research) - Global Forecast to 2027"
View detailed Table of Content here - https://www.marketsandmarkets.com/Market-Reports/in-vitro-diagnostics-quality-controls-market-198032582.html
The growth of the IVD quality control market is driven by the rising number of accredited clinical laboratories, Rising geriatric population and subsequent growth in the prevalence of chronic and infectious diseases and increasing adoption of third-party quality controls. The rising focus on multi-analyte controls and Increasing investments from government bodies and private players in healthcare sectors in emerging economies is also expected to offer significant growth opportunities for the market in the coming years. Lack of regulations for clinical laboratory accreditation in several emerging countries could be the challenges faced by the market in upcoming years.
The product & service segment holds the highest share of the total IVD quality control market during the forecast period.
The quality control products segment accounted for the largest share of the IVD quality control market in the base year. The increasing number of accredited laboratories and mandates for the use of quality controls from regulatory bodies are driving the growth of the IVD quality control products market. The growth in the number of IVD tests performed, the increased number of accredited laboratories, and the implementation of mandates from regulatory bodies for ensuring the accuracy of diagnostic test results using QC materials are also some of the major factors influencing this market growth.
Immunochemistry accounted for the highest share of the technology segment of the global IVD quality control market
The immunochemistry segment accounted for the largest share of the IVD quality control market. The large share of the immunochemistry segment can be attributed to the growing use of immunoassay controls in diagnostics. However, the molecular diagnostics segment is estimated to grow at the highest CAGR of 7.9% during the forecast period, majorly due to the rising prevalence of infectious diseases such as tuberculosis, influenza, pneumonia, and COVID-19.
Third-party controls accounted for the largest share for the IVD quality control market
Based on manufacturer, the IVD quality control market is segmented into third-party controls and OEM controls. The third-party controls segment accounted for the largest share of the global IVD quality control market. The large share of this segment is mainly attributed to the increasing use of third-party quality controls due to their benefits, such as longer shelf-life and flexibility across different reagent lots, which helps reduce costs. Other benefits include unbiased independent assessment, consistent analyte-level stability, and clinically relevant levels.
Hospitals accounted for the highest share of the global IVD quality control market
The hospitals segment accounted for the largest share of the IVD quality control market, owing to the large volume of diagnostic tests carried out in hospitals. Clinical laboratories are estimated to be the fastest-growing end-user segment in this market. Amid the COVID-19 pandemic, manufacturers developed a diverse range of controls for clinical laboratories, hospitals, and research centers, as ensuring the accuracy of tests is vital to curb the spread of the outbreak.
Based on Region, North America to dominate the global IVD quality control market
North America dominated the global IVD quality control market. Approvals of quality control products from the FDA and the College of American Pathologists (CAP) and the presence of leading companies in the US are driving the IVD quality control market in North America. US dominated the North America IVD quality control market. In the coming years, the increasing number of IVD tests; the growing need to ensure the accuracy, reliability, and reproducibility of test results; and the use of third-party quality controls among clinical laboratories in this region will play a key role in the market growth.
Some of the key players in the market include Bio-Rad Laboratories, Inc. (US), Randox Laboratories Ltd. (UK), Thermo Fisher Scientific, Inc. (US), LGC Limited (UK), and Abbott Laboratories (US). Other prominent payers in the market include Roche Diagnostics (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), Fortress Diagnostics (UK), SERO AS (US), Sysmex Corporation (Japan), Ortho-Clinical Diagnostics (US), Helena Laboratories Corporation (US), Quidel Corporation (US), Sun Diagnostics, LLC (US), Seegene Inc. (South Korea), ZeptoMetrix Corporation (US), Qnostics (UK), Bio-Techne Corporation (US), Microbiologics (US), Microbix Biosystems (Canada), Streck, Inc. (US), Alpha-Tec Systems (US), Maine Molecular Quality Controls, Inc. (US), and Grifols, S.A. (Spain).
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their painpoints around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model – GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 MicroQuadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, "Knowledgestore" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA : 1-888-600-6441
sales@marketsandmarkets.com